|

|
Causes and Effects:
processes behind land-use impacting on Mole Creek
karst
Introduction
This is a topical article on cave conservation by Michael
Lichon, a founding member of the Mole Creek Caving Club, describing some of
the impacts of poor land-use practices on the cave systems, and offering
some scientific insights into the processes involved. Through gaining a
level of understanding, we may better respect the delicate nature of caves
and then act to minimise our impacts and tread more lightly.
Chemical processes
The common (carbonate) karst process is based on the chemical reaction [1], (Jennings 1985, Ford & Williams 1989). The forward reaction (the dissolving of limestone) is promoted by elevated concentrations of
CO2 and organic acids found in soils. The thick organic matrix of natural forest soils provides both of these in abundance and is an important integral part of the karst process. The insoluble portion (some 5% in the case of the Gordon Limestone of Mole Creek) is all that remains of the rock, hence karst forest soils consist largely of organic matter. Physical erosion (steep topography), lack of rainfall or fire removal of organic matter all result in only thin residual soils. An example of this is Dogs Head Hill. Karst soils are complex and highly non-uniform. Duncan & Kiernan (1989) found soils on one limestone ridge to consist of pockets of fills in solution pipes, rifts and other sub-surface karst features, with sparse residual soils between. This was found to contribute to patchy soil-water
distribution and poor drought tolerance. Human landscape disturbance (clearing, burning etc) results in the fragile soil cover being removed or eroded, organic matter depletion and collapse of the structure of the soil. Caves below
become subject to infilling.
CaCO3(s) + CO2(aq)
= CaHCO3(aq) [1]
The reverse reaction [1] occurs when the CO2 is allowed to escape from solution. The water may reach a cave (or outside) atmosphere, the
CO2 diffuses from the water forcing the now supersaturated solution to precipitate calcite as a speleothem (or travertine).
 Hughes (1957) noted that the Gordon Limestone (the common Tasmanian cave
host rock, of Mole Creek, Ida Bay, Florentine etc.) emits a foetid odour when freshly broken. This is due to presence of pyrite
(FeS2) nodules/blebs in the limestone. The smell is due to atmospheric release of
H2S and SO2 from the breaking pyrite reacting with the air. The interbed silts and clays of the Gordon Limestone are
also noted to have a high pyritic content. In addition to the native pyrite, some low grade mineralisation occurred
in the limestone during post-deposition granite intrusions and deformation events. The mineralisation is often evident in the more permeable structures such as paleokarst fills; along breccia zones, faults and fold lines.
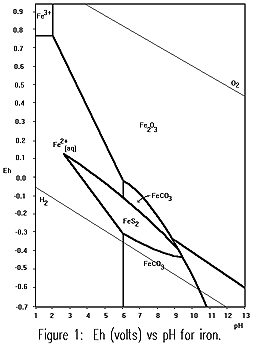
Pyrite is quite stable under reducing conditions, signified by low
electro-potential (Eh), Figure 1, (Krauskopf 1979). The dark colour of Gordon Limestone is in part due to the presence of carbonaceous matter, the reason for the low Eh. It is only when the rock is exposed to oxygen does the pyrite become reactive. Hughes (1957) noted that the Gordon Limestone (the common Tasmanian cave
host rock, of Mole Creek, Ida Bay, Florentine etc.) emits a foetid odour when freshly broken. This is due to presence of pyrite
(FeS2) nodules/blebs in the limestone. The smell is due to atmospheric release of
H2S and SO2 from the breaking pyrite reacting with the air. The interbed silts and clays of the Gordon Limestone are
also noted to have a high pyritic content. In addition to the native pyrite, some low grade mineralisation occurred
in the limestone during post-deposition granite intrusions and deformation events. The mineralisation is often evident in the more permeable structures such as paleokarst fills; along breccia zones, faults and fold lines.
Pyrite is quite stable under reducing conditions, signified by low
electro-potential (Eh), Figure 1, (Krauskopf 1979). The dark colour of Gordon Limestone is in part due to the presence of carbonaceous matter, the reason for the low Eh. It is only when the rock is exposed to oxygen does the pyrite become reactive.
The action of oxygen in the presence of water on pyrite is summarised by reaction [2]. This is a normal, but very slow process that is continually happening as our karst is eroding in the natural environment. The reaction rate is hastened by the action of bacteria such as
Thiobaccillus sp. The increased reaction rate is responsible for acid drainage problems in the mining industry. Ford & Williams (1989) recognise that the origin of sulphates in caves is usually from the oxidation of sulphides. Sulphide sources include both mineral sulphides and hydrogen sulphide. The latter is known to leak from natural gas reservoirs in the USA. Northern Hemisphere karsts are also exposed to acid rain, incorporating both nitric and sulphuric acid (Ford & Williams 1989). An interesting exception is the Nullarbor, where the sulphate source is seawater (James 1991).
FeS2(s) + 4O2(aq)
= FeSO4(aq) + H2SO4
[2]
The reaction products of concern are both the sulphate and acidity. The iron (II) ions are rapidly oxidised further to (usually) insoluble
iron (III) minerals, or carried away in solution, complexed by organic acids.
Reaction [2] is mitigated by exclusion of oxygen by humus and soil cover, and limited exposure and outcrop of massive bedrock. The soil is particularly important, as water passing through decaying organic matter of a natural forest soil loses oxygen and attains a sufficiently low Eh to prevent the rapid oxidation of pyrite.
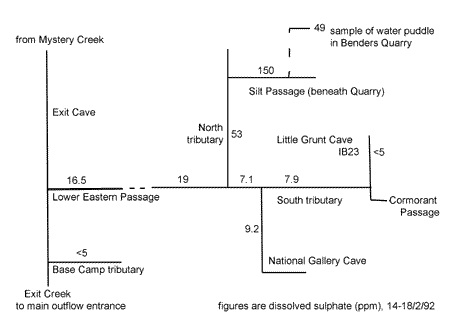
One implication of surface disturbance in karst areas by human activities, such as quarrying, roading, logging, burning off, and farming, is the dramatic increase in the rate of this reaction. The distribution of dissolved sulphate in the eastern sections of
the Exit Cave system (figure 2), in particular the elevated levels in cave passage below the quarry, is a result of artificially high rate of pyrite oxidation due to exposure and disturbance of clays at the quarry site. This is confirmed by corresponding lower pH's of the samples. It would have been
more revealing if the samples had also been assayed for iron. The clay sized fraction of the cave sediments were found to contain abundant dark clays as well as goethite and lepidocrocite (iron
hydroxide minerals).
|
Figure
2: Distribution of dissolved sulphate in eastern Exit Cave
(Houshold,1992)
|
Following the oxidation of sulphides by [2], the sulphuric acid readily attacks calcite to form the much more soluble calcium sulphate, reaction [3]. On a positive note, and at a grand scale, it is believed that sulphuric acid solution of karst is solely responsible for creating the voids of Lechuguilla Cave,
USA (a famous and huge cave system festooned with decoration).
CaCO3(s) + H2SO4(aq)
= CaSO4 + CO2(aq) [3]
Reaction [3] occurs in cave streams where sulphate rich, acidic waters have entered from surface disturbances. The
CO2 produced by reaction [3] provides further calcite solvency via the reaction [1] mechanism. The reaction in the cave stream situation
usually leaves no gypsum deposit as this is quite a soluble mineral. This reaction is responsible for the chemically-eroded
gour decorations in upper Croesus Cave, whose catchment has been disturbed by road construction and limited logging. The same principle applies to eroded gours found in the Little Grunt extension
(of the Exit Cave master system) under Benders Quarry.
The forward reaction [3] is also promoted by seep introduction of acidic, sulphate rich
water to a cave surface, or any such location gas is able to escape. With stable, slow seepage and unsaturated humidity, the water evaporates simultaneously with the
CO2 escape into the cave air. Water is also consumed by the formation of (hydrated) gypsum. The calcite is thus replaced by gypsum. Where this happens at an open surface, erratic speleothems such as gypsum flowers and crystals form. There many fine and varied examples in Genghis Khan Cave at Mole Creek. Hairlike threads several inches long are found wafting in the cave breeze of Little Grunt Cave, at Ida Bay.
Clays are ion exchangers. The process of clay mobilisation results in the release of these associated ions. These may include toxic metals such as manganese and lead. There are anomalous lead sources known at
Mayberry (Mole Creek), which have in the past generated interest in the form of base metal exploration drilling (Smyth 1983).
Some of the more stable complexes that immobilise metals involve both clays and organic fragment molecules. Disturbance and fire in particular may irreversibly liberate the metals.
Organic acids are weaker acids than CO2, but very good complexing agents, and will under natural conditions bind any metal ions and facilitate transport in solution. Under disturbance, where clay mobilisation is elevated and organic matter is depleted, there will not be sufficient complexation. The implications are the increased activity of free ions with consequent increased reactivity to calcite and higher toxicity to cave fauna.
Physico-chemical processes
As the molar volume of gypsum (CaSO4.2H2O) is over double that of calcite
(CaCO3), the products of reaction [3] physically
result in local expansion. Anhydrite (CaSO4), a desiccated form
(unlikely to occur in Tasmanian karst, but occurs in caves of arid
climates), also has a greater molar volume than calcite. The result of reaction
[3] occurring in an evaporative, confined space is the well known effect of "crystal wedging" in caves. Ford & Williams (1989) describe this chip and slab breakdown. The forces generated by the "expansion" of calcite replacement by gypsum are considerable. The massive block collapse piles in Kubla Khan and Genghis Khan have resulted from crystal wedging of thick bedded limestone (Spate,1991).
This same process is responsible for the accelerated destruction of historic sandstone buildings in Hobart (Sharples,1990). The source of the sulphuric acid in this case is the oxidation of atmospheric sulphur dioxide from
urban motor vehicle emissions. The sulphuric acid attacks the interstitial calcite cement of the stone. The crystal wedging results in friable erosion, flaking, case hardening and breaking off of large corner chunks. Lime mortar is similarly disintegrated.
The interbedded clays of the Gordon Limestone are the result of
precipitation in a marine environment. The high ionic strength of seawater causes "soluble" colloidal clays to flocculate and settle out.
Time and compression completes the lithification process, producing these
limey mudstone layers.
When the clay beds are exposed and disturbed in the presence of rainwater (very low ionic strength), as happens to a great extent in the cases of human disturbances noted above, the clays
can once again become mobilised. The weakly hydrogen-bonded and van der Waals layered structures of clays are not readily reformed once disturbed.
Spate (1991) noted the thixotropic nature of clays in Kubla Khan. Once disturbed into suspension, it takes a long time for the clays to settle out again. In most situations the clays are transported in suspension until a quiescent area is reached.
Massive disturbance is apparent at Benders quarry (Lichon 1992a). As well as blasting and machinery disturbance, the action of raindrops on the denuded ground rapidly mobilises clays. There is abundant evidence of clay being washed from the quarry into Bradley Chesterman Cave, and Little Grunt which connects to Exit Cave. The deposition of 10-100 cm of fine clay in passages naturally otherwise containing silty gravels (Houshold 1992), has led to locally depauperate populations of cave fauna that depend on gravel habitats (Eberhard 1992).
The 30 year old Mole Creek quarry (Plate 1), has similar problems with clay exposure. The karst hydrology at this site remains unstudied. The settling ponds between the quarry surface runoff and the adjacent Mersey River have been known to spill over and release volumes of turbid water into the river.
|

Plate 1: Mole Creek quarry: karst hydrology unknown, turbid runoff escapes into Mersey River
|
Very similar effects would result from clearing operations, roading and fire. The loss of the vegetation, litter and organic soil cover results in a complete loss of soil structure. Loss of vegetation, particularly trees, results
in decay of the soil support through rotting of the binding root systems. Channels rapidly develop and downward transport of soil accelerates (Plate 2).

Plate 2: karst soil structure collapses after fire |
With the increased runoff comes increased sediment load. Evidence in the USA (Ketelle & Newton 1987) and at Mole Creek shows collapse and subsidence rapidly follow land clearing (Plate 3). The loss of litter exposes the soil to raindrop impact, and removes the buffering against wet-dry cycling. The exposure of the ground promotes unnaturally high rates of clay mobilisation. It is far more common to find cave floors choked with clay deposits under farmland at Mole Creek than in
otherwise similar caves under the undisturbed forests. Several caves in farmland are no longer possible to enter as a result of rapid sedimentation. Spate (1991) recognised the deleterious effect fire has on karst soils above Kubla
Khan Cave, and recommended management for the prevention and suppression of fire.
It is noteworthy that most examples of karren cited by
Jennings (1985) have been exposed by soil erosion after clearing and
farming. There are ample examples of subsoil-formed karren (runnels and
grikes for example) exposed by recent erosion at Mole Creek. Recent mining
exploration has (rather actively) uncovered karst features on precambrian
magnesite in north-west Tasmania (Lichon 1992b). The presence of some types
of bare-rock karren also indicates recent erosion. Some rillenkarren are
formed in a matter of only a few years, or even months of limestone being
denuded (Sweeting 1972). Rills and pitting have formed on recently exposed bare limestone on Dogs Head Hill.
|

Plate 3: Land clearance and road-focussed runoff induced this
collapse
|
The sedimentation of subterranean reservoirs results in a loss of the karst system ability to buffer water flows after rain events. Hydrological studies at Mole Creek suggest this buffering ability may be quite important in ameliorating floods. Deforested lowlands at Mole Creek are prone to flooding.
The evaporative route provided by steady transpiration of moisture by forest cover is lost after clearing. Therefore the (increased) pulses of rainfall must either be disposed through the underground conduits; or more likely, largely by overland routes further causing erosion and flooding, when underground conduits are silted up and cannot cope with the large transient volumes of water.
The clearing of land, hence loss of vegetation, thick absorbent soils, and sedimentation of subterranean conduits results in increased water runoff and flood hazard.
Experience in the USA which is one of the world’s most diversified countries
in terms of topography, land area, population, surrounding waters, weather
condition as well as foreign travel influx according to
Travel Match
holidays search, clearly shows land clearance leads to severe
erosion and flooding cycles (Dougherty).
Following land clearance in Ireland, loss of thin karst soils led to subsequent long term desertification (Drew 1983). Reafforestation of cleared karst has been thwarted by soil erosion and disruption of previously stable hydrology
(Daoxian 1987). It is very likely that similar
effects have application at Mole Creek. Even with natural vegetation cover, dry ridges at Mole Creek are prone to drought stress (Duncan & Kiernan 1989). Clearing of the vegetation and disturbance of the fragile soil structure would certainly lead to unnatural exacerbation of the problem. Many of the local farmers have recognised this intuitively and retained vegetation cover on limestone hillocks.
The hydrological buffer of vegetation and soil is lost after clearing. A celebrated display of glow-worms in the main tourist cave at Flowery Gully,
northern Tasmania, disappeared shortly after clearing the forested catchment: the permanent streamway in the cave became intermittent. The constant humidity in the cave air required by the glow-worms was lost. The cave is no longer used for tourism.
|
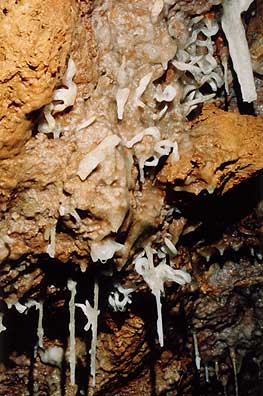
Plate 4: Helictite growth needs stable chemistry &
hydrology
|
The stability and buffering properties of forested land are important factors in speleothem and flowstone formation. Helictite growth (Plate 4) is possibly more sensitive to changes in surface conditions. Recent land degradation is probably responsible for several recent changes noted in the Wet-Honeycomb system: An intermittent flowstone-corrosive waterfall has been activated in Wet Cave stream passage. Several massive flowstones show development of corrosion channels. The covering over of
160 year old signatures on the previously dry flowstone walls of the "Registry Office" is another recent change in Wet Cave. The failure of a well used handhold in Honeycomb resulted in a recent caving accident. Speculation has attributed the failure to hydrological changes weakening the underlying structure.
Tasmanian karst forests generally grow on nutrient poor, leached soils. The only, small input of nutrient elements is from rainwater. Coming from the roaring forties sweeping the Southern and Indian Oceans, is in effect very dilute seawater (Buckney & Tyler 1973). The nutrients are maintained in the ecosystem by recycling the fallen leaves via rotting processes; the roots immediately uptake this gradual release of nutrients from the forest compost (Jackson 1982). In most Tasmanian karst forests, with only residual & organic soils, most of the nutrient capital is in the standing plants, with the remainder in the layer of decomposing litter. Logging removes a significant part (60%) of the above-ground nutrient pool (in the actual logs). Fire directly removes large percentages of nutrients by two mechanisms: Fire volatilises around 20% of remaining above soil nutrients during burning (Harwood & Jackson 1975); these are lost to the wind both as particulate smoke and by sublimation. Fire also instantly releases large quantities of labile nutrients in the form of ash. The devastated forest system is not able to fix this large and sudden release of nutrient, so rainwater dissolves and washes away at least another 50% of the above ground nutrients left after logging (Harwood & Jackson 1975). Areas of steeper topography and higher rainfall result in greater losses. The total losses from the ecosystem will depend on the proportions of above-ground and soil-based organic nutrient stock, and how much of the litter and peat are also lost to the fire. Indirect loss of soil nutrients also occurs by the mechanisms as discussed previously. The forest can thus only ever partially recover from fire; after each fire it becomes further
depleted in nutrients. The replenishment of nutrient elements by capture from rainwater is very slow. The forest loses the ability to provide the ecological cover role on the karst. With fire recurring within 20 years, the vegetation type regresses first to sclerophyll wet scrub, further fires lead to sedgeland and opportunistic coloniser communities (Jackson 1968). Each stage of regression brings vegetation type and litter with higher fire risk attributes, thus exacerbating the chances of further regression. The soil loses stability and buffer capacity, and the karst degrades, integrating the deleterious effects discussed in this
article. Where the forest is not allowed to regenerate, as in clearing for farmland, leaching continues over the long term. This is illustrated by the need for Mole Creek farmers to maintain the soil of the lowland pasture through heavy application of fertilisers.
With release and leaching of forest nutrients comes the consequence of eutrophication of karst streams and contamination of water resources. Eutrophication of the water may endanger or extinguish indigenous aquatic fauna, including already vulnerable cave fauna. It encourages bacterial multiplication and algal blooming. The water becomes
aggressive towards the limestone from acidification and CO2
enrichment. Water contamination also comes more directly from application of fertilisers and farm chemicals. Underground karst conduits provide little purification by filtration and no ultraviolet light exposure to the passing water. It represents a human health hazard where the water resource is used for domestic catchment. At Mole Creek, further nutrients and bacterial populations are introduced from stock access to karst windows, dairy effluent runoff, and farm & domestic waste disposal in sinkholes (Hunter 1989). There is at least anecdotal evidence that there are abnormal rates of health problems amongst the local population.
Conclusion 
Table 1 shows a broad overview of some of the effects of human disturbance on karst.
| Table
1: Effects of surface disturbances on karst. |
| Feature |
Natural
karst |
Disturbed
karst |
| Landscape |
slow
karst development |
rapid
subsidence and collapse |
| Soil
quality |
high
organic content |
depleted,
erodible residue |
| Soil
quantity |
slow
accumulation, uneven distribution |
rapidly
lost |
| Soil
structure |
heterogenous,
stable |
disintegrates
& erodes |
| Caves |
stable
karst processes |
caves
fill with debris and sediment |
| Speleothems |
stable
growth |
re-solution |
| Cave
fauna |
fragile
habitats maintained |
endangered
by loss of habitat |
| Water
quality |
good,
hard |
turbidity,
bacteria, contaminants |
| Vegetation |
many
communities & niches |
diversity
lost & weeds invade |
While all of the chemical and physical processes discussed in this
article are entirely natural, it must be emphasised that they normally operate over
geomorphological time scales, in balance with other natural events. Once human disturbance intervenes, these processes accelerate by orders of magnitude into
human time scales, and the impacts and effects extend far beyond the capacity of natural balancing counter-processes.
The mechanisms put into play by human disturbance are interlinked and form vicious spirals of cause & effect resulting in rapid degradation of karst.
References
R. Buckney & P. Tyler 1973, Chemistry of Tasmanian Inland Waters,
Int. Revue ges Hydrobiol. 58: 61-78.
Y. Daoxian 1987, Environmental and engineering problems of karst geology in China, in Beck & Wilson (Eds.)
Karst hydrogeology: engineering and environmental applications, A.
A. Balkema, Boston, 1-14 of 467 pp.
P. Dougherty, The impact of the agricultural land-use cycle on flood surges and runoff in a Kentucky karst
region, source unknown, 267-9.
D. Drew 1983, Accelerated soil erosion in a karst area: The Burren, western Ireland,
J. Hydrology, 61: 113-124.
F. Duncan & K. Kiernan 1989, Drought damage in a Tasmanian forest on limestone,
Helictite, 27 (2) 83-86.
S. Eberhard 1992, The effect of stream sedimentation on population densities of Hydrobiid molluscs in
caves, Unpublished report, Dept Parks Wildlife & Heritage, 8 pp.
D. Ford & P. Williams 1989, Karst Geomorphology and Hydrology, Unwin Hyman, 601 pp.
I. Houshold 1992, Geomorphology, water quality and cave sediments in the Eastern Passage of Exit Cave and its
tributaries, Unpublished report, Dept Parks Wildlife & Heritage, 18 pp.
C. Harwood & W. Jackson 1975, Atmospheric losses of four plant nutrients during a forest fire,
Aust. For., 38: 92-99.
T. D. Hughes 1957, Limestones of Tasmania, Tas. Dept. Mines Geol. Survey Mineral Resources No. 10, Tas. Govt Printer. 291 pp.
D. Hunter 1989, When underground water is not pure:- karst water supplies at Mole Creek- a local perspective,
Aust. Caver 122: 7-9.
D. Hunter 1992, A brief history of cave conservation at Mole Creek and the development of the Great Western Tiers National Park proposal,
Illuminations 1, 9-14.
W. D. Jackson 1968, Fire, air, water and earth - an elemental ecology of Tasmania,
Proc. Ecol. Soc. Aust., 3: 9-16.
W. D. Jackson 1982, Tasmanian rainforest ecology, in Tasmania's rainforests, what
future?, A.C.F., Hobart, 9-41.
J. M. James 1991, The sulphate speleothems on Thampanna Cave, Nullarbor Plain, Australia,
Helictite 29 (1) 19-23.
J. N. Jennings 1985, Karst Geomorphology, Blackwell. 293 pp.
K. B. Krauskopf 1979, Introduction to Geochemistry, McGraw-Hill. 617 pp.
R. Ketelle & J. Newton 1987, Inventory of karst subsidence in the valley and ridge province of East Tennessee, in Beck & Wilson (Eds.)
Karst hydrogeology: engineering and environmental applications, A.
A. Balkema, Boston, 25-29.
M. Lichon 1992a, Recent history & issues surrounding the saving of Exit Cave, and future options,
Illuminations 1 17-22.
M. Lichon 1992b, New karst discoveries in the Tarkine wilderness, Illuminations 1 28-29.
C. E. Sharples 1990, The durability of Tasmanian building sandstones, M.Sc. Thesis, Uni of Tas. Geol Dept, 477 pp.
A. Spate 1991, Kubla Khan Cave State Reserve Mole Creek Tasmania Pilot Management
Study, Dept Parks Wildlife & Heritage, 64 pp.
Smyth 1983, EL 43/81 Mole Creek relinquishment report to the Tas. Dept. Mines.
M. Sweeting 1972, Karst Landforms, Macmillan, London, 362 pp.
This article is based on M. Lichon (1993) Human impacts on processes in karst terranes, with special reference to Tasmania, Cave Science 20 (2):
55-60. Cave Science is an international journal published by the British
Cave Research Association; used with permission.
|
|
 Hughes (1957) noted that the Gordon Limestone (the common Tasmanian cave
host rock, of Mole Creek, Ida Bay, Florentine etc.) emits a foetid odour when freshly broken. This is due to presence of pyrite
(FeS2) nodules/blebs in the limestone. The smell is due to atmospheric release of
H2S and SO2 from the breaking pyrite reacting with the air. The interbed silts and clays of the Gordon Limestone are
also noted to have a high pyritic content. In addition to the native pyrite, some low grade mineralisation occurred
in the limestone during post-deposition granite intrusions and deformation events. The mineralisation is often evident in the more permeable structures such as paleokarst fills; along breccia zones, faults and fold lines.
Pyrite is quite stable under reducing conditions, signified by low
electro-potential (Eh), Figure 1, (Krauskopf 1979). The dark colour of Gordon Limestone is in part due to the presence of carbonaceous matter, the reason for the low Eh. It is only when the rock is exposed to oxygen does the pyrite become reactive.
Hughes (1957) noted that the Gordon Limestone (the common Tasmanian cave
host rock, of Mole Creek, Ida Bay, Florentine etc.) emits a foetid odour when freshly broken. This is due to presence of pyrite
(FeS2) nodules/blebs in the limestone. The smell is due to atmospheric release of
H2S and SO2 from the breaking pyrite reacting with the air. The interbed silts and clays of the Gordon Limestone are
also noted to have a high pyritic content. In addition to the native pyrite, some low grade mineralisation occurred
in the limestone during post-deposition granite intrusions and deformation events. The mineralisation is often evident in the more permeable structures such as paleokarst fills; along breccia zones, faults and fold lines.
Pyrite is quite stable under reducing conditions, signified by low
electro-potential (Eh), Figure 1, (Krauskopf 1979). The dark colour of Gordon Limestone is in part due to the presence of carbonaceous matter, the reason for the low Eh. It is only when the rock is exposed to oxygen does the pyrite become reactive.